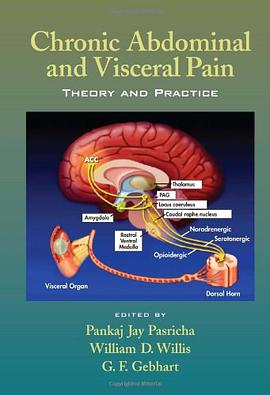
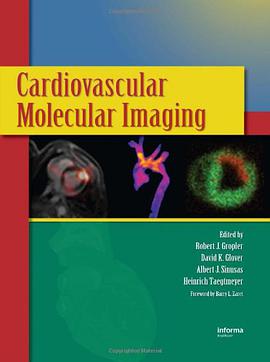
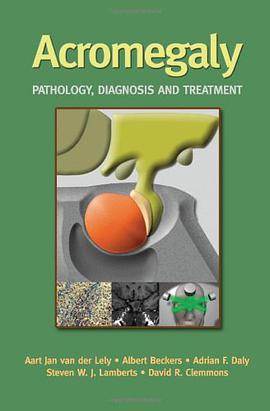
具体描述
"Safety Evaluation: International Regulations for Drug and Medical Device Approval" analyzes the international drug laws and regulations that pertain to safety and prescribes how safety procedures can be implemented. This text describes the development process for products in various markets, outlining those aspects of drug and device regulation related to evaluating safety. Presenting a review of the major regulatory bodies responsible for approval of new products, particularly in the US, EU, and Japan, this source also provides a clear understanding of the regulation requirements and includes current synthesis of disparate regulations. Features:
作者简介
目录信息
读后感
评分
评分
评分
评分
用户评价
相关图书
本站所有内容均为互联网搜索引擎提供的公开搜索信息,本站不存储任何数据与内容,任何内容与数据均与本站无关,如有需要请联系相关搜索引擎包括但不限于百度,google,bing,sogou 等
© 2026 onlinetoolsland.com All Rights Reserved. 本本书屋 版权所有