Good Manuf Pract for Phar 2025 pdf epub mobi 電子書 下載

簡體網頁||繁體網頁
Good Manuf Pract for Phar pdf epub mobi 著者簡介
Good Manuf Pract for Phar pdf epub mobi 圖書描述
With global harmonization of regulatory requirements and quality standards and national and global business consolidations ongoing at a fast pace, pharmaceutical manufacturers, suppliers, contractors, and distributors are impacted by continual change. Offering a wide assortment of policy and guidance document references and interpretations, this Sixth Edition is significantly expanded to reflect the increase of information and changing practices in CGMP regulation and pharmaceutical manufacturing and control practices worldwide. An essential companion for every pharmaceutical professional, this guide is updated and expanded by a team of industry experts, each member with extensive experience in industry or academic settings.
Good Manuf Pract for Phar pdf epub mobi 圖書目錄
點擊這裡下載
發表於2025-01-06
Good Manuf Pract for Phar 2025 pdf epub mobi 電子書 下載
Good Manuf Pract for Phar 2025 pdf epub mobi 電子書 下載
Good Manuf Pract for Phar 2025 pdf epub mobi 電子書 下載
喜欢 Good Manuf Pract for Phar 電子書 的读者还喜欢
Good Manuf Pract for Phar pdf epub mobi 讀後感
圖書標籤:
Good Manuf Pract for Phar 2025 pdf epub mobi 電子書 下載
Good Manuf Pract for Phar pdf epub mobi 用戶評價
Good Manuf Pract for Phar 2025 pdf epub mobi 電子書 下載
分享鏈接


Good Manuf Pract for Phar 2025 pdf epub mobi 電子書 下載
相關圖書
-
 Accountability 2025 pdf epub mobi 電子書 下載
Accountability 2025 pdf epub mobi 電子書 下載 -
 Foot and Ankle Motion Analysis 2025 pdf epub mobi 電子書 下載
Foot and Ankle Motion Analysis 2025 pdf epub mobi 電子書 下載 -
 Clinical Management of Chronic Obstructive Pulmonary Disease 2025 pdf epub mobi 電子書 下載
Clinical Management of Chronic Obstructive Pulmonary Disease 2025 pdf epub mobi 電子書 下載 -
 Selfish Mr. Mermaid 2025 pdf epub mobi 電子書 下載
Selfish Mr. Mermaid 2025 pdf epub mobi 電子書 下載 -
 Zhi zhuo di zhui qiu (Mandarin Chinese Edition) 2025 pdf epub mobi 電子書 下載
Zhi zhuo di zhui qiu (Mandarin Chinese Edition) 2025 pdf epub mobi 電子書 下載 -
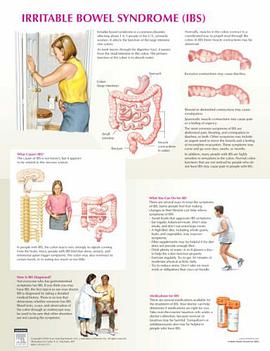
 Irritable Bowel Syndrome Chart 2025 pdf epub mobi 電子書 下載
Irritable Bowel Syndrome Chart 2025 pdf epub mobi 電子書 下載 -
 Getting the Best Out of College 2025 pdf epub mobi 電子書 下載
Getting the Best Out of College 2025 pdf epub mobi 電子書 下載 -
 Disrobe, Completely 2025 pdf epub mobi 電子書 下載
Disrobe, Completely 2025 pdf epub mobi 電子書 下載 -
 Pasta 2025 pdf epub mobi 電子書 下載
Pasta 2025 pdf epub mobi 電子書 下載 -
 Toxicokinetics and Risk Assessment 2025 pdf epub mobi 電子書 下載
Toxicokinetics and Risk Assessment 2025 pdf epub mobi 電子書 下載 -
 365 Bible Stories for Young Hearts 2025 pdf epub mobi 電子書 下載
365 Bible Stories for Young Hearts 2025 pdf epub mobi 電子書 下載 -
 Diagnostic and Surgical Imaging Anatomy 2025 pdf epub mobi 電子書 下載
Diagnostic and Surgical Imaging Anatomy 2025 pdf epub mobi 電子書 下載 -
 Mural Painting Secrets For Success 2025 pdf epub mobi 電子書 下載
Mural Painting Secrets For Success 2025 pdf epub mobi 電子書 下載 -
 Diagnostic and Surgical Imaging Anatomy: Brain, Head & Neck, Spine Ebook License Key 2025 pdf epub mobi 電子書 下載
Diagnostic and Surgical Imaging Anatomy: Brain, Head & Neck, Spine Ebook License Key 2025 pdf epub mobi 電子書 下載 -
 Tracing Punctuation! 2025 pdf epub mobi 電子書 下載
Tracing Punctuation! 2025 pdf epub mobi 電子書 下載 -
 Free the Market! 2025 pdf epub mobi 電子書 下載
Free the Market! 2025 pdf epub mobi 電子書 下載 -
 The Field Guide to Safari Animals 2025 pdf epub mobi 電子書 下載
The Field Guide to Safari Animals 2025 pdf epub mobi 電子書 下載 -
 全世界都在玩的智力遊戲 2025 pdf epub mobi 電子書 下載
全世界都在玩的智力遊戲 2025 pdf epub mobi 電子書 下載 -
 全世界都在玩的科學遊戲 2025 pdf epub mobi 電子書 下載
全世界都在玩的科學遊戲 2025 pdf epub mobi 電子書 下載 -
 全世界都在玩的科學遊戲 2025 pdf epub mobi 電子書 下載
全世界都在玩的科學遊戲 2025 pdf epub mobi 電子書 下載